Company is first to demonstrate restoration of vision in clinical trial of BCI tech in patients with geographic atrophy.
A clinical trial published in The New England Journal of Medicine has demonstrated that Science Corporation’s brain-computer interface (BCI) retinal implant can restore functional central vision to patients with an advanced form of age-related macular degeneration (AMD), one of the leading causes of blindness worldwide. The publication is the first peer-reviewed confirmation that patients with geographic atrophy regained so-called “form vision” – the ability to perceive shapes, letters and words – through a wireless retinal prosthesis.
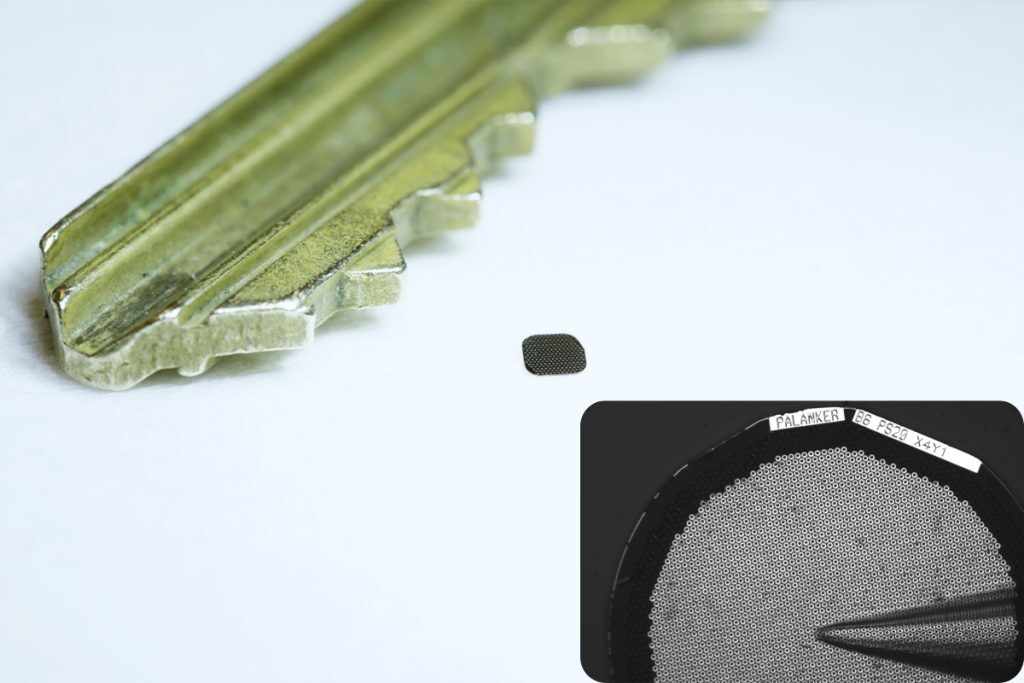
It is estimated that more than 5 million people globally suffer from geographic atrophy, a late-stage form of AMD that destroys the photoreceptors responsible for sharp, detailed vision. Until now, no treatment has been able to restore lost sight in patients with the condition. The technology is based on the work of Stanford professor of ophthalmology Daniel Palanker.
“All previous attempts to provide vision with prosthetic devices resulted in basically light sensitivity, not really form vision,” said Palanker, a co-senior author of the paper, in a statement. “We are the first to provide form vision.”
The multi-center trial enrolled 38 patients at 17 sites across five countries, evaluating the safety and effectiveness of the device. Participants in the trial had severe central vision loss due to geographic atrophy. After implantation and visual training, most patients were able to read letters, numbers, or words, restoring a level of functional vision previously thought unattainable for this population.

“It’s the first time that an attempt at vision restoration in these cases has achieved results – and in such a large number of patients,” said University of Pittsburgh professor of ophthalmology José-Alain Sahel, senior co-author of the paper. “More than 80% of the patients were able to read letters and words, and some of them are reading pages in a book. This is really something we couldn’t have dreamt of when we started on this journey together with Professor Palanker more than a decade ago.”
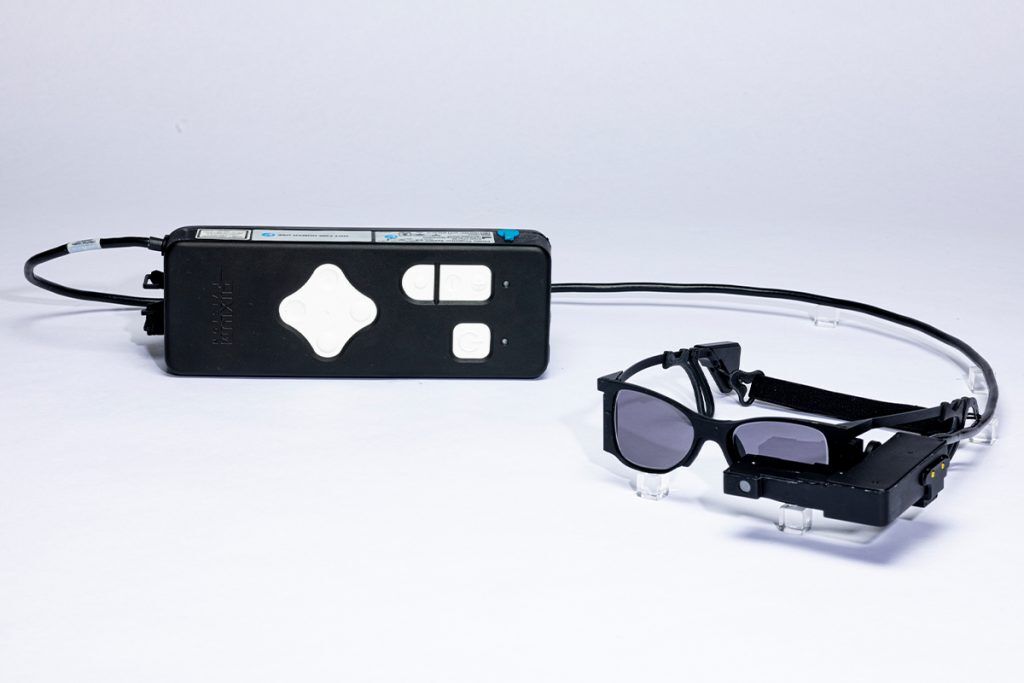
The PRIMA system was refined by Science Corp, a California-based company specializing in neural engineering and BCI technologies. The implant consists of a 2-by-2-millimeter photovoltaic chip that replaces the function of lost photoreceptors, coupled with a pair of glasses that project infrared light patterns onto the retina. These patterns are converted into electrical signals that stimulate the remaining retinal cells, which then relay visual information to the brain.
PRIMA’s design leverages the transparency of the eye to transmit visual information wirelessly. The implant, just 30 microns thick, operates as a miniature solar panel, powered by near-infrared light emitted from the glasses. Patients can adjust contrast and magnification, and the system allows prosthetic central vision to integrate with their natural peripheral vision, enabling users to read text and navigate their surroundings.
Current PRIMA devices deliver black-and-white vision, although Science Corp. says development is underway to introduce grayscale perception and higher resolution through smaller pixels and enhanced image processing.
The trial also confirmed that the implant could be safely placed beneath the atrophic macula. The Data Safety Monitoring Board concluded that the therapeutic benefits outweighed the risks, recommending PRIMA for European regulatory approval. Science Corp. has now submitted its application and expects the device to become available to patients in Europe next year, while the US FDA review process continues.


