
Apple snail research sheds light on how complex eyes can regenerate – and why human ones can’t, yet.
When the eyes go, so too – often – does confidence, independence and much of what makes the world navigable. The slow erosion of vision, whether by trauma, degenerative disease or age, is one of the most feared and frustrating aspects of human aging. So news of a species capable of regenerating not just retinal tissue but an entire camera-type eye – and doing so with surgical completeness – invites astonishment followed by a cascade of scientific questions.
Enter Pomacea canaliculata, the freshwater apple snail. More usually condemned for its invasiveness than celebrated for its biomedical credentials, this gastropod is now setting a brisk research pace, despite its leisurely locomotion. In a new paper published in Nature Communications, researchers at the Stowers Institute for Medical Research detail how this small mollusk can regenerate an entire functional eye within four weeks of full amputation – complete with lens, retina, optic nerve and cornea – and how its genome can now be edited with precision thanks to newly established CRISPR protocols [1].
Longevity.Technology: Snails may not be the first creatures that spring to mind when considering the future of regenerative medicine – but perhaps that’s the point. With the apple snail now emerging as a genetically tractable model for whole-eye regeneration, researchers may have just added a new tool to the aging biology arsenal – and not a moment too soon. Human eyes, so fundamental to quality of life and independence in later years, are frustratingly poor at repairing themselves; the ability of this humble mollusk to regrow a camera-type eye – lens, retina, cornea and all – invites fresh inquiry into why regeneration fails in us, but not in them. Given the prevalence of age-related vision loss and the lack of true curative treatments, the snail’s ocular party trick is far from a biological curiosity – it may be the beginning of a roadmap to structural repair for one of aging’s most disabling and intractable burdens.
That the research team achieved stable genome edits in this unlikely system is, frankly, impressive; CRISPR in snails is not the usual Tuesday in the lab. Having both a regenerative phenotype and a manipulable genome makes Pomacea canaliculata unusually powerful as a discovery engine – the fact that it comes with ready-made evolutionary proximity to human eye anatomy is almost showing off. But perhaps the most enduring lesson here is that we still have a great deal to learn from life forms that don’t make the cover of Nature; betting on underdog organisms may yet be one of the smartest plays in longevity science. After all, the next therapeutic breakthrough might not come from billion-dollar screens in mice, but from a slow-moving undersnail with excellent eyes and absolutely no heed for scientific protocol.
The research team, led by Stowers’ President Dr Alejandro Sánchez Alvarado, sought an animal that could provide both regenerative capacity and genetic manipulability – a rare pairing. Apple snails, they found, offered both: diploid genomes, year-round fertility, camera-type eyes and, crucially, amenability to gene editing and stable mutant line development.

“Our eyes are extremely important for perceiving our environment, yet when damaged are unable to recover,” said lead author Dr Alice Accorsi.
“Essentially we had no way to identify solutions for treating conditions like retinal degeneration or physical injury to the human eye,” added Sánchez Alvarado. “But nature has answers for us. We now have a tractable system for investigating which genes are responsible for camera-type eye regeneration.”
Better optics
Previous work in regeneration has typically featured flatworms, zebrafish and salamanders – creatures capable of regrowing tails, limbs or even neural tissues. In ocular regeneration specifically, some fish and amphibians can repair discrete components of the eye, such as the retina or lens, but not the organ in its entirety. Planarians, meanwhile, can regenerate simple pigment-cup eyes but lack the structural complexity of camera-type systems. As the paper’s authors note, the Pomacea model now bridges a significant gap.
Importantly, the apple snail eye shares several key anatomical and molecular features with vertebrate eyes, including photoreceptor organisation and expression of genes associated with camera-type eye morphogenesis – among them pax6, a master regulator of eye development in flies, mice and humans alike. The team not only confirmed the presence of a functional pax6 ortholog in the snail, but demonstrated its essential role by generating pax6-null mutant lines via CRISPR – the resulting embryos developed normally apart from having no eyes whatsoever.
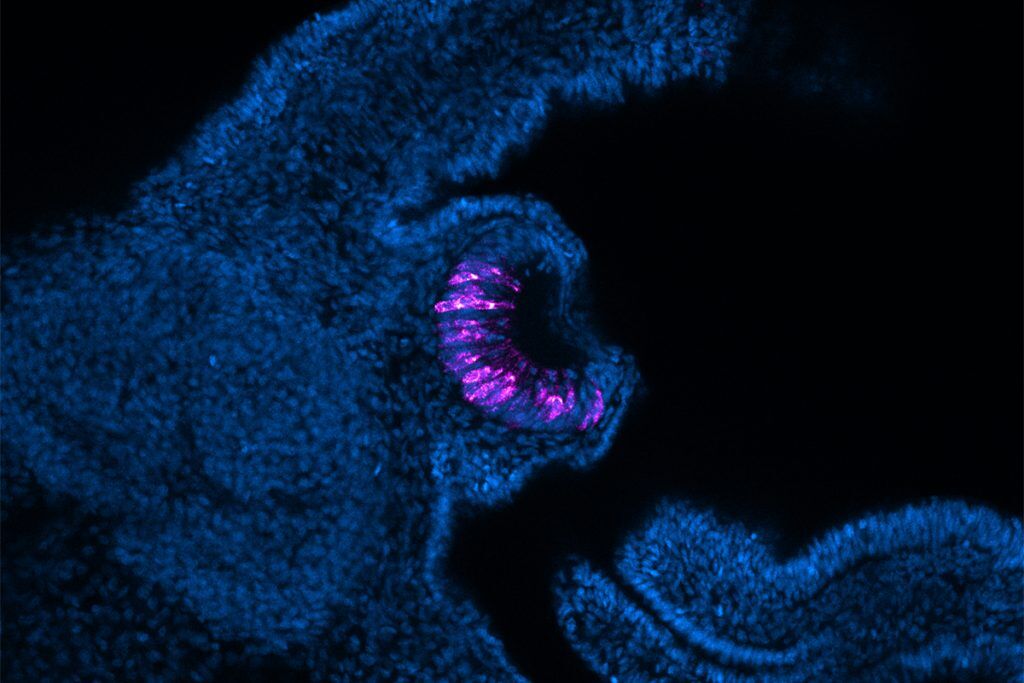
According to the authors, the regeneration process proceeds through four distinct stages: wound healing, blastema formation, emergence of retina and lens, and subsequent maturation of all eye components [1]. Transcriptional profiling across these phases revealed gene expression dynamics that closely mirror developmental pathways, suggesting that regeneration may be, at least in part, a recapitulation of embryogenesis – a valuable clue for therapeutic translation.
The apple snail doesn’t fall far from the translation tree
With gene-editing techniques established and candidate genes identified, the Pomacea model opens the possibility of comparative studies that ask why regeneration is retained in some species but lost in others. In an aging population increasingly affected by vision loss – macular degeneration, glaucoma, diabetic retinopathy – a system that combines regenerative biology with genetic accessibility is more than welcome.
The paper’s authors also highlight the broader implications of working with unconventional models [1]. While mice and zebrafish remain staples of the lab, their limitations in regeneration are well known. By bringing Pomacea into the fold, the researchers have not only expanded the phylogenetic spread of model organisms but also demonstrated the value of scientific risk-taking. “With a little bit of effort, a little bit of ingenuity, and a great deal of persistence, biology that seemed inaccessible is no longer a pipe dream,” Sánchez Alvarado said. “Our work with the apple snails is proof positive – it really is possible to bring something that was far beyond what we thought we could do into the realm of real possibility to advance biological knowledge.”
Slow science, fast potential
It remains to be seen whether the apple snail will join the elite ranks of mainstream model organisms or remain a specialist system for regeneration studies. But the trail it blazes is already instructive: regeneration may not require reinvention, just recognition – and the willingness to follow nature’s less obvious paths.
[1] https://www.nature.com/articles/s41467-025-61681-6


