Coya Therapeutics is pioneering multi-pathway therapies for ALS, Alzheimer’s and other complex aging-related conditions.
In the rapidly evolving landscape of therapeutic development, Houston-based Coya Therapeutics has emerged as a proponent of combination biologics in the treatment of neurodegenerative diseases and conditions associated with aging. The company’s strategic approach involves targeting multiple pathways simultaneously, diverging from the traditional single-target drug development model. This methodology aims to address the complex mechanisms underpinning conditions like ALS, Alzheimer’s and other inflammatory and neurodegenerative diseases.
Longevity.Technology: Combination biologics are gaining traction as a potentially key strategy in tackling these multifaceted conditions. By addressing multiple disease-driving pathways concurrently, they offer a more nuanced approach to managing the intricate interplay between neurodegeneration, inflammaging and systemic immune response. This approach holds promise in overcoming the limitations of conventional treatments, which can often fall short when it comes to managing the complexities of such diseases.
Coya’s performance in this domain has been marked by a dedication to advancing the understanding of regulatory T cells (Tregs) and their role in neurodegenerative disease progression. Its lead asset, COYA 302, represents the culmination of this research focus, employing a dual mechanism of action to enhance Treg function while mitigating inflammatory mediators. Additionally, the company’s recent patent filing for the combination of COYA 301 and GLP-1 receptor agonists signals an expansion in their pipeline, suggesting a broader application of their combination biologic strategy. We sat down with Coya Therapeutics’ CEO Dr Howard Berman to find out more.
Coya’s rationale is that the traditional ‘one disease – one target – one drug’ approach might not be the most appropriate framework for neurodegenerative disorders and may even be partly responsible for the lack of available and truly effective treatments.
“What is unique to our approach is that we recognize that neurodegeneration involves complex mechanisms, in addition to dysfunctional regulatory T cells (Tregs), that are responsible for driving disease pathophysiology,” Berman explains, adding that Coya Therapeutics’ lead asset is a combination of two biologic drugs that target multiple disease-driving pathways.
“The immune system plays a critical role in health and disease, and our own research has established the key role of the immune system, especially Tregs in the pathogenesis of progressive neurodegenerative diseases,” he adds. “COYA 302 is our lead biologic combination asset that aims to enhance the number and function of Tregs while reducing other inflammatory mediators and ultimately ensuring that Tregs remain durably functional. COYA 302 is a combination of two drugs including COYA 301 (Coya’s proprietary LD IL-2) and CTLA4-Ig for subcutaneous administration with a unique dual mechanism of action that is now being developed for the treatment of Amyotrophic Lateral Sclerosis (ALS), Alzheimer’s disease, Frontotemporal dementia (FTD), and Parkinson’s disease (PD).”
The key role of Tregs in autoimmune conditions has been well documented; these severe and potentially fatal conditions have limited treatment options, highlighting, as Berman points out, the clear and urgent unmet need.
“Our mission is to restore the immune balance by enhancing Treg function, resulting in suppression of systemic and neuro-inflammation and modifying the course of these devastating conditions.”
Coya is backing the belief that combination immunotherapy approaches will evolve to play a meaningful role in treating complex immune-based diseases, that are driven by a host of pathophysiologic mechanisms. Its recent patent filing for the combination of COYA 301 and GLP-1 receptor agonists marks a significant expansion of its pipeline, with the COYA 301/GLP-1 RA combination targeting multiple, independent and non-overlapping immune pathways simultaneously.
Berman explains that this aligns with Coya’s combination R&D strategy as seen with COYA 302, which is the combination of COYA 301 or LD IL-2 and CTLA-4 Ig.
“GLP-1 RAs and LD IL-2 act through distinct mechanisms of action to exert anti-inflammatory and Treg-enhancing effects and may make this multi-targeted therapeutic approach an appealing combination to potentially address the unmet needs of patients with severe systemic and neuro-inflammatory, autoimmune, and metabolic conditions,” he says, adding that such dynamic and complex conditions may benefit from the combination treatments that address multiple pathophysiological pathways simultaneously.
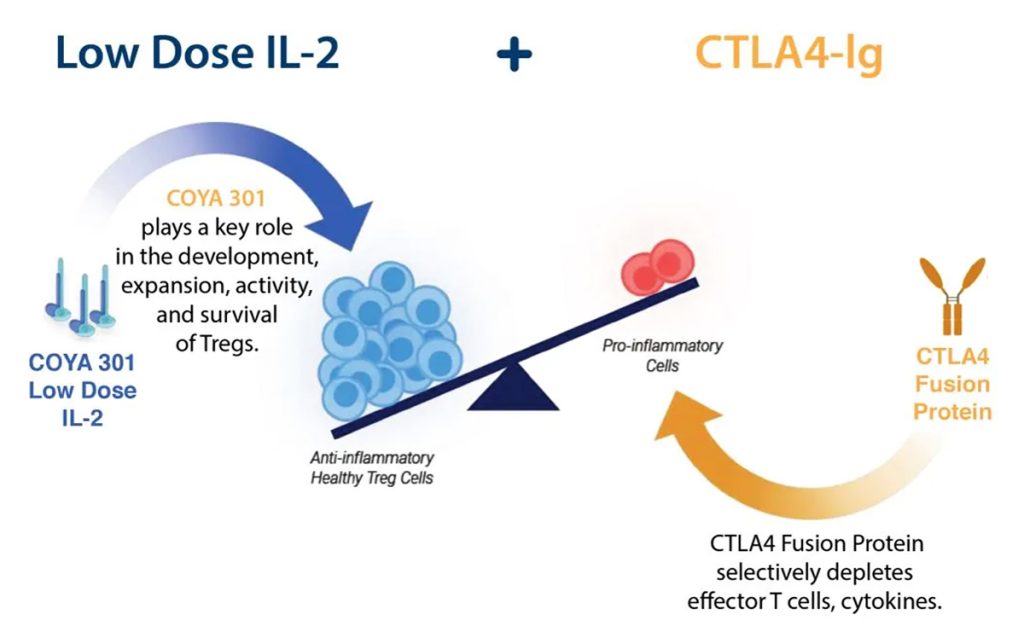
LD IL-2 is a cytokine essential for the enhancement of Treg function and numbers and which suppresses inflammatory responses, while GLP-1 RAs possesses neuroprotective and anti-inflammatory properties via modulation of microglial activity, reduction of oxidative stress and promotion of neuronal survival.
“We will continue to expand our portfolio with additional synergistic drug combinations with COYA 301 as we see potential,” says Berman.
Others are seeing the potential in Coya Therapeutics’ approach – the recent $5 million investment from the Alzheimer’s Drug Discovery Foundation (ADDF) is a notable endorsement, and Berman says the Coya team is very excited about the funding, which is earmarked for a planned phase 2 trial of COYA 302 in Frontotemporal Dementia (FTD).
“The ADDF’s commitment to helping us fund this important trial and their particular awareness to the potential of combination biologics like ours has been nothing short of inspirational,” says Berman. “We hope to show in that phase 2 trial that COYA 302 has the potential to provide benefit to patients with FTD through the targeting of multiple immune pathways.”
In neurodegenerative diseases, the suppressive function and number of circulating Tregs are decreased. Berman explains that this Treg dysfunction results in increased inflammation and, consequently, disease progression.
“Our data shows that in patients with ALS, more marked Treg dysfunction is associated with faster progression and severity,” he explains. “Similarly, Treg dysfunction is observed in patients with other neurodegenerative diseases and observed in serious autoimmune conditions.”
Coya’s goal is to restore immune balance by enhancing the function of Tregs and fighting the inflammatory cascade produced by other types of immune cells, and its core assets are biologics that repair the Treg dysfunction but simultaneously block other pro-inflammatory pathways; this ultimately results in synergistic approaches that may keep Tregs durably functional, active and viable.
“The key discovery we have made is that inflammation is an important and central driver of these diseases and that Treg cell biology is the common denominator,” says Berman. “That is why we are enthusiastic about this approach, as it can have overarching implications for a wide variety of indications across neurodegenerative, metabolic, and autoimmune diseases.”
It is a busy time for Coya Therapeutics; as well as gearing up to file the IND for COYA 302 in ALS and subsequently initiate the phase 2b clinical trial, the company plans to share important data from a double-blind, placebo-controlled study in Alzheimer’s patients at the Clinical Trials on Alzheimer’s Disease Conference (CTAD) in late October.
“The data of that study will determine next steps for our Alzheimer’s program but also aim to validate the role of Treg modulation in neurodegenerative diseases,” explains Berman. “Over the mid-term, we expect to have the data readout from that phase 2b ALS trial with COYA 302 and are looking forward to other regulatory milestones ahead.”
In parallel with the phase 2b ALS trial, there is the aforementioned phase 2 trial in patients with FTD, and Coya plans to share data from that in 2025/6.
Berman explains that the phase 2 data will guide the company’s next steps, including the potential initiation of a subsequent registrational study in FTD, assuming the phase 2 data supports continued development. The FTD indication will also bring additional potential partnership opportunities for Coya.
The company also has other assets in its pipeline which it plans to advance; these include its exosome-based programs which Berman explains could be ideal for multiple partnering opportunities.
There is also progress on a strategic level for Coya as well, as it was recently announced that Berman will be stepping down as CEO and moving to a role as Executive Chairman of the Board; current Chief Business Officer Arun Swaminathan will be taking his place.
“That is very exciting for the company going forward as we begin to understand how we take these important medicines from investigational targets to commercialized therapeutics to widely available treatment for patients.”


