Ochre Bio launches Liver ICU, where drug candidates can be tested in real human organs under physiological conditions.
This week, Ochre Bio, a biotech focused on chronic liver disease, launched a new perfusion laboratory in New York that aims to push the boundaries of translational research. Located in the Alexandria Center for Life Sciences, the first-of-its-kind facility, dubbed the “Liver ICU,” is designed to maintain human donor livers in near-physiological conditions while testing the company’s RNA-based therapeutic candidates in real time.
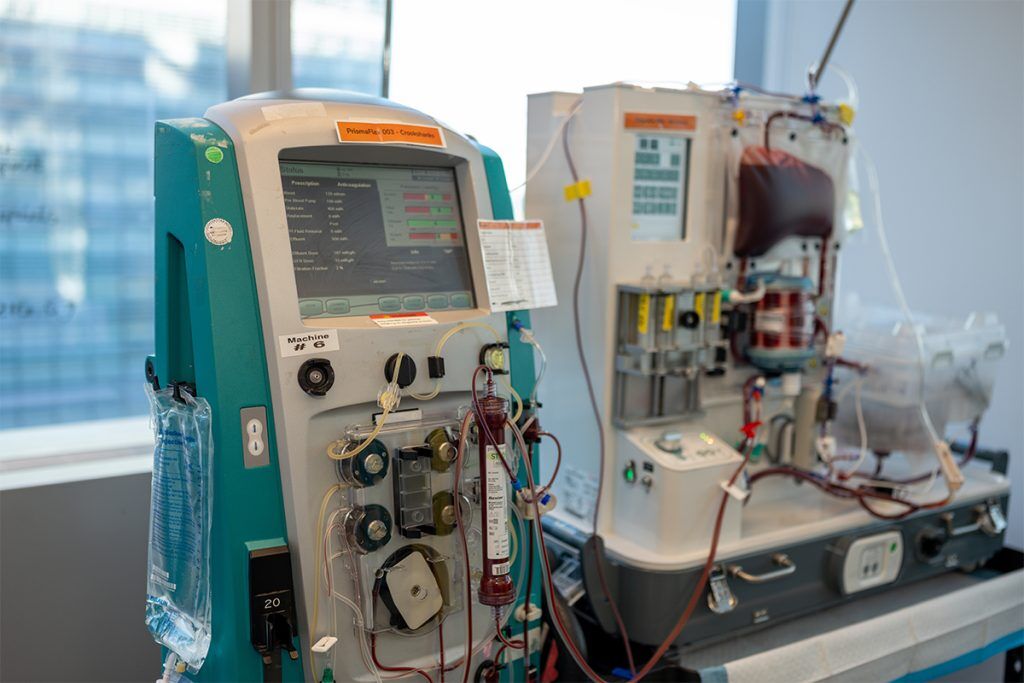
Ochre Bio describes its approach as running a “clinical trial before the clinical trial,” effectively generating human efficacy data at the preclinical stage. Designed to study drug effects directly in living human organs outside the body, the lab can run multiple simultaneous perfusions, connecting donor livers to machines that circulate warm, oxygenated, nutrient-rich blood.
The setup allows Ochre scientists to observe organ-level metabolic and regenerative responses to RNA treatments in a controlled environment. By replicating the functional state of the liver after removal from the body, the platform aims to bridge a gap in the traditional drug development process that often relies on animal models to predict human outcomes and sees up to 90% of drug candidates fail in clinical trials.
Longevity.Technology: Co-founded by physician-scientist Quin Wills, Ochre Bio was built on the idea that studying real human liver tissue – rather than animal surrogates – would produce more predictive biological insights and accelerate therapeutic discovery. This philosophy evolved into a “human-first” approach, using deep phenotyping, machine learning and large-scale human datasets to identify and validate the company’s therapeutic RNA targets. With the launch of the Liver ICU, the company’s human-first vision has evolved one step further, and we sat down with Wills to learn more.
The underpinning idea behind Ochre Bio is that studying real human liver tissue – rather than animal models – can unlock new insights into human liver pathophysiology and drive faster, more predictive drug discovery.

“Pharma has traditionally been chemistry-first,” says Wills. “Many companies are essentially large chemistry organizations, with biology as an afterthought. That’s changing. We’re moving toward biology-first drug discovery.”
“The key lesson, learned from oncology and elsewhere, is that if you want to model and predict real long-term human impact, you need not only to generate more human data and run great clinical trials – you need a human platform for validation. That’s our philosophy.”
Overcoming the ‘valley of death’
True to its founder’s vision, Ochre’s lab in Oxford, UK, works exclusively with primary human cell models to study the basic mechanisms of disease, while its Taiwan facility is the only lab in the world that cultures diseased human liver tissue at scale.
“And now here in New York, we’ve established the only lab in the world that keeps whole human livers alive,” says Wills. “This is our final ‘stage gate,’ where we test therapies and ideas – complex ones, not just fibrosis, but also regeneration.”
“A typical liver trial can easily cost $100 million, and the history of the field is full of failures because animal models just don’t predict human outcomes,” he adds. “The Liver ICU bridges that gap. It allows us to study complex processes like fibrosis and regeneration directly in human organs, reducing that ‘valley of death’ between preclinical work and human trials.”
Founded in 2019, Ochre has raised over $110 million in funding, including $40 million in equity investment, alongside more than $70 million in strategic and non-dilutive financing. The firm has also established long-term collaborations with Big Pharma, including GSK and Boehringer Ingelheim.
“Our seed funding was focused on building foundational human datasets,” says Wills. “Early on, we performed the first large-scale spatial sequencing of 1,000 human livers, mapping gene expression and histology at high resolution, and linking that to blood biomarkers and patient outcomes. We’ve since expanded on that, including a major AI collaboration with GSK where we knocked out every gene in the liver – across multiple donors and disease states – and sequenced everything to map gene function to outcome.”
‘Remarkably detailed insights’
Following its Series A funding, Ochre focused on building out its experimental platforms, and the Liver ICU represents a scaling up of that process.
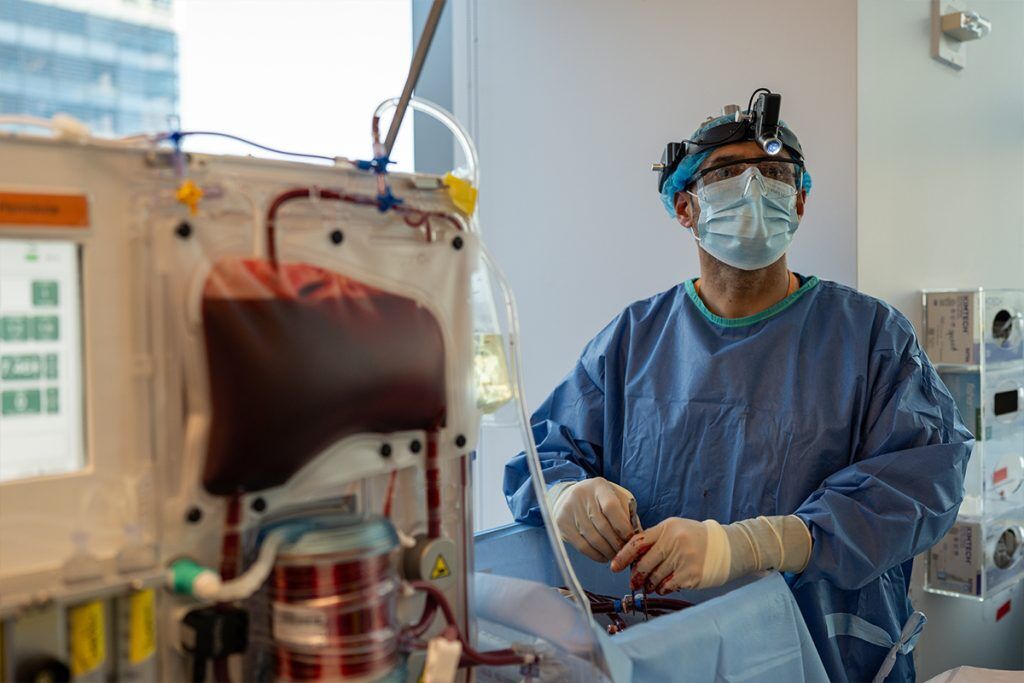
“We’ve moved from a small lab on the edge of Manhattan to a full-scale ICU,” says Wills. “We have three surgical teams on rotation, keeping livers on machines, collecting biopsies, culturing tissues, and running around a thousand perturbations per liver.”
While the idea of keeping organs alive on machines isn’t new – perfusion devices have been around for some years now – Wills explains that doing it at scale, and keeping human livers alive for five days or more, is extremely rare.
“There are academic and commercial groups doing similar work with animal organs, but our setup is different: it’s a dedicated ICU, running multiple human livers simultaneously, for extended periods, under tightly controlled conditions,” he says. “We’re effectively running mini clinical trials before the clinical trial. The ability to do that reproducibly and generate high-quality data is what makes this unique – and it’s taken years of refinement to get right.”
Ochre works with US organ procurement organizations, relying on the generosity of donors and their families. While livers are first considered for transplant cases, sometimes a liver that was offered for transplantation can’t be used.
“Perhaps it’s too fatty, or there’s no suitable recipient – those are the livers we receive,” says Wills. “We run a 24-hour facility with surgical teams on standby. Once a liver arrives, it’s connected to the perfusion machine, and the specific study protocol determines what happens next. For example, we can then test how our RNA therapies influence regeneration over five to ten days. With a cohort size of around 10–20 per arm, we get remarkably detailed insights.”
Clinical trials on the horizon
The next step for Ochre is moving toward the clinic and raising funds for its first development candidate – a therapeutic RNA program that is expected to enter IND-enabling studies next year.
“Our lead drug candidate is incredibly effective at making liver cells resistant to death – essentially giving them time to repair and regenerate,” says Wills. “In later stage liver disease, fibrosis and regeneration become the critical balance. Liver failure isn’t just about scarring – it’s about the organ’s ability to regenerate and maintain function. Our view, also shared by others, is that therapies must address both.”
Of course, Ochre’s Liver ICU won’t just be used for its own internal programs. The company has already been successful at inking partnerships with the likes of GSK and Boehringer Ingelheim on various liver-related programs, and it’s clear that the strategy is set to continue.
“Watch this space – we’re about to announce another major pharma partnership in the next few weeks,” teases Wills. “This new collaboration will focus more explicitly on obesity and liver-mediated effects. The liver is a central regulator of metabolism – it affects everything from weight and glucose control to muscle gain. So we’re designing studies that explore those systemic connections.”


