New paper argues broad impact of GLP-1s supports the development of therapies that target entire systems rather than isolated dysfunctions.
A new paper in the British Journal of Clinical Pharmacology sets out framework for a new generation of drugs designed to chronic disease and aging by targeting the body’s biological systems as interconnected networks. Entitled Beyond GLP-1s: The Blueprint for Systemic Therapeutics That Will Reshape Aging and Medicine, the paper outlines a strategy for “system-level” medicines delivering the multi-organ benefits of GLP-1 receptor agonists while avoiding their trade-offs.
The success of GLP-1 drugs such as Ozempic and Wegovy has already transformed how medicine views complex metabolic diseases. Indeed, more than one Big Pharma representative referred to GLP-1s as “longevity drugs” at the recent ARDD conference in Copenhagen. Initially developed for type 2 diabetes, these drugs have since demonstrated powerful effects on weight loss and revealed unexpected improvements across cardiovascular, renal and neurological health.
The results show how a single therapy designed to modulate hormone signaling pathways that coordinate metabolism can influence multiple organs at once – highlighting the potential of system-level interventions to improve health and longevity.

“Traditionally, medicine and drug discovery has treated diseases as separate entities – one drug for one condition,” said the paper’s lead author Dr Carina Kern. “Open even the most recent medical textbook, and you’ll find separate chapters on cardiovascular disease, kidney disease, neurological disorders.
“But biology doesn’t work that way. The body functions as an interconnected network, and the success of GLP-1s shows what happens when you target the system rather than the symptom.”
Target ‘network-level’ processes
Kern, whose Blueprint Theory of Aging predicted the broad systemic impact of GLP-1s back in 2023, teamed up on the new paper with a host of authors, including leading aging researchers Tina Woods and Richard Faragher, to argue that GLP-1s mark only the first phase of a broader therapeutic paradigm.
By acting on shared biological systems, the authors propose that targeting network-level processes can treat not just symptoms but underlying disease mechanisms. Of course, the same systemic reach also creates risks, as interventions affecting multiple organs can disrupt the body’s finely balanced homeostasis. The authors propose a new generation of precision systemic therapies that sustain the benefits of GLP-1s while maintaining equilibrium across biological systems.
“GLP-1 drugs have shown us that targeting the biological system as a whole can improve health across multiple disease and organ systems,” said Woods, Executive Director at the International Institute of Longevity. “The next challenge and opportunity is to design new therapies that achieve the same systemic benefits, but with greater precision and fewer side effects.”
The review examines how GLP-1 receptor agonists and their successors extend their influence beyond glucose and lipid metabolism to vascular biology, inflammatory signaling and energy regulation. The findings point to the feasibility of developing combination drugs that recalibrate entire physiological networks rather than simply correct isolated dysfunctions.
Restorative versus curative
The authors argue that long-standing disease classifications based on lipid or glucose abnormalities are increasingly outdated. Instead, metabolic disorders like obesity, non-alcoholic steatohepatitis and type 2 diabetes should be viewed as states of systemic dysregulation within the neuro-endocrine network linking the brain, gut, liver and adipose tissue. Future therapeutics, they suggest, should aim to restore this signaling architecture through interventions that integrate advances in pharmacokinetics, receptor modelling and metabolic imaging. Such “restorative” rather than “curative” pharmacology could address not only chronic metabolic conditions but also the wider processes of aging that underlie them.
The paper identifies necrosis – the uncontrolled death of cells and tissues – as one of the most promising biological targets for the next phase of drug discovery. By focusing on necrosis, researchers hope to develop drugs that preserve tissue integrity across a range of conditions, from cardiovascular and kidney disease to neurodegeneration.
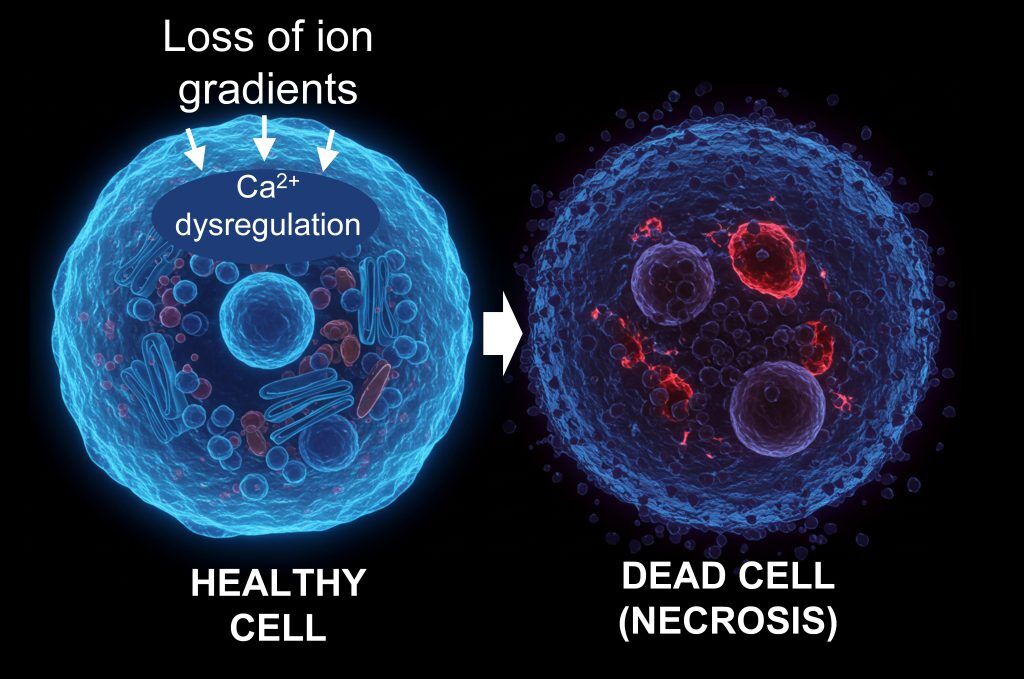
“The beauty of necrosis is that, unlike genetically programmed biological pathways, necrosis is an unprogrammed process – meaning blocking it comes with significantly lower risk of unwanted compensatory effects elsewhere in the body,” said Imperial College London professor and leading oncologist Justin Stebbing, also a co-author on the paper. “If GLP-1s were version 1.0 of systemic medicine, necrosis inhibitors could represent version 2.0.”
Next up: necrosis
The necrosis concept is already being taken forward by LinkGevity, a biotechnology company co-founded by Kern. Guided by its founder’s Blueprint theory, the company applies AI to map the molecular “patho-pathways” that drive aging, integrating semantic data analysis and computational modelling to identify systemic biological triggers and predict therapeutic targets.
“Think of it this way: biology is complex and interlinked, like an electricity grid,” said Kern. “Within the grid, what we are looking for is key nodes that transmit the greatest level of destruction across the system. We set ourselves a task – can we identify another node like GLP-1s in the grid?”
LinkGevity’s flagship program is an anti-necrotic therapeutic designed to inhibit uncontrolled cell death and enhance tissue resilience. Its lead candidate is being developed for kidney-related tissue degeneration, with a first clinical trial expected in the coming months.
The company’s program has also been selected for the NASA/Microsoft Space Health initiative, using spaceflight as a model to accelerate insights into tissue decline, since microgravity and radiation amplify the same biological processes that drive aging on Earth. Its anti-necrotic approach is being tested through a UK–Lithuania collaboration with Delta Biosciences, funded by the UK Space Agency and the Lithuanian Health Ministry, to explore potential applications for both astronaut health and terrestrial medicine.


